by Aubrey Rumore
John, 22, from San Antonio, TX, is the first and only patient enrolled in a groundbreaking treatment protocol for Aplastic Anemia at the National Institutes of Health (NIH) Clinical Center. He remembers well the day he was diagnosed. It was the fall of 2015. He was on his way to his little brother’s middle school football game when his right leg went numb. “I didn’t think anything of it,” he said, “It’s like when your foot ‘falls asleep’.”
But not long after John sat down at the football game, he, his mother, and his one-year-old son, Gavin, were headed to the ER. John had lost feeling in the right side of his face, his mouth drooped and he couldn’t talk. Tests soon revealed that he had zero platelets, dangerously low hemoglobin and low white blood cell counts – all classic signs of Aplastic Anemia.
When John failed traditional treatment for the disease a year later, he researched alternatives himself which led him to a mother of young man with the disease. She pointed out trials at the NIH. John took the initiative to contact an NIH research nurse, sent tubes of blood and was soon accepted into a study.
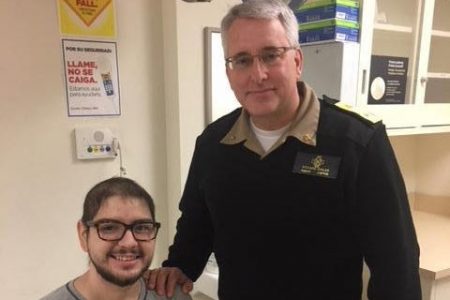
John and Dr. Richard Childs
Luckily, as the oldest of six kids, John had a sibling donor, his brother Isaiah, which made him eligible for a transplant although the match was not perfect. After testing and delays, his brother was cleared to be the donor, but then NIH called to say the trial had closed.
But, “it was actually a good thing,” John said, because with this trial closed, an even more promising transplant protocol was made available. In this new trial, John would receive cells from an umbilical cord blood unit that had stem cells expanded in the laboratory for 3 weeks, combined with mismatched or haplo-identical stem cells from his brother. Cord blood transplants allow patients who lack a perfectly matched donor an option for transplantation, but conventional cord-blood transplants contain very limited number of stem cells. By multiplying the cells 100-fold in the novel stem cell expansion technique, the chances of graft failure are lowered and the growth speed of new, healthy blood cells is increased.
John’s lead physician at the NIH, Rear Admiral Richard Childs, Assistant U.S. Surgeon General and Clinical Director of the National Heart, Lung, and Blood Institute (NHLBI) says the transplant has been a great success and paves the way for future patients. “His blood counts came back quicker than any patient we’ve ever seen, faster than patients with traditional transplants from a sibling. He’s the first on this trial and he’s a trailblazer.”
Dr. Childs also reports that the good news for other Aplastic Anemia patients from the early success of this study is that cord cell transplants are more readily available than sibling matches and “as an off the shelf stem cell product that does not have to be perfectly matched…. the expanded cord blood transplant John received may someday become a first line treatment that is not experimental.”
John agrees the unique cord blood transplant “worked fantastically,” but his weak immune system made him highly susceptible to viruses. He needed a place to stay near the NIH that provided minimal contact with sick people. Thanks to generous donors, Friends of Patients at the NIH provided John and his sister Isabella, 21, who serves as his primary caregiver, with an apartment where they could stay free of charge.
“I’m really grateful for what we have in place,” John said. Without the apartment, John feels it would be very difficult to cover basic expenses like food. Without this option, “I would still be at home,” John said, “with a failed transplant or no treatment at all.” Now after 180 days of treatment and monitoring, John can’t wait to return home this week to see his son, and enjoy Mexican food at his favorite restaurant.
About Aplastic Anemia
Aplastic Anemia is a blood disorder in which the body’s bone marrow doesn’t make enough new blood cells. Treatment includes blood transfusions, blood and marrow stem cell transplants and medicines. ClinicalTrials.gov currently lists 19 open studies for patients with Aplastic Anemia. Visit ClinicalTrials.gov.